Graphene quantum dots (GQDs) refer to a new type of carbon based fluorescent material with a graphene layer size of less than 100nm and a number of layers of less than 10. Generally speaking, graphene quantum dots include a large class of carbon fluorescent materials and their derivatives with similar structures and properties, including graphene quantum dots, oxidized graphene quantum dots, and partially reduced oxidized graphene quantum dots.
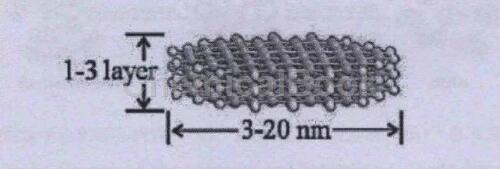
The Properties of Graphene Quantum Dots
UV absorption performance of graphene quantum dots
Due to the C=C double bond structure in graphene quantum dots, π - π transitions can occur, allowing them to absorb a large number of photons in a short wavelength range. Generally speaking, a strong absorption peak is displayed in the range of 260-320nm in the UV absorption spectrum, accompanied by a tailing that extends to the visible light range. Meanwhile, due to the influence of n - π transitions, graphene quantum dots may also exhibit shoulder peaks in the range of 270-390nm. Moreover, due to the influence of surface modification functional groups and surface passivation, the position and shape of the UV absorption peak will be affected.
Photoluminescence properties of graphene quantum dots
The luminescence performance of graphene quantum dots is its most important performance, and it is also the most widely studied and practical performance by researchers. Compared to spherical carbon quantum dots, graphene quantum dots with a layered structure have a more regular crystalline structure, resulting in higher fluorescence quantum yields.
Synthesis of Graphene Quantum Dots
There are two methods for preparing graphene quantum dots: top-down and bottom-up.
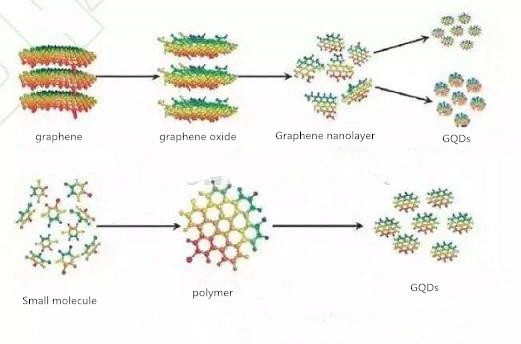
Top-down synthesis
The top-down approach refers to the physical or chemical etching of large-sized materials into nanoscale graphene quantum dots, which can be prepared through solvent thermal, electrochemical, and chemical exfoliation pathways.
The solvent thermal method is one of the many methods for preparing graphene quantum dots, and its process can be divided into three steps: first, the oxidized graphene is reduced to graphene nanosheets under high temperature in a vacuum state; Oxidize and cut graphene nanosheets in concentrated sulfuric acid and concentrated nitric acid; Finally, the oxidized graphene nanosheets are reduced in a solvent thermal environment to form graphene quantum dots.
The process of electrochemical preparation of graphene quantum dots can be summarized into three stages: the stage is the induction period when graphite is about to peel off and form graphene, and the color of the electrolyte begins to change from colorless to yellow and then to dark brown; The second stage is a significant expansion of graphite in the anode; The third stage is when the graphite flakes have peeled off from the anode and formed a black solution together with the electrolyte. In the second and third stages, sediment was found at the bottom of the beaker. In electrochemical reactions, there is an interaction between water and anions in ionic liquids, so the shape and size distribution of the products can be adjusted by changing the ratio of water to ionic liquids. The size of quantum dots prepared from electrolytes with high ion concentration is larger than that of electrolytes with low concentration.
The principle of chemical exfoliation of carbon fibers is to exfoliate the carbon source layer by layer through chemical reactions to obtain graphene quantum dots. Peng et al. used resin based carbon fibers as the carbon source, and then peeled off the graphite stacked in the fibers through acid treatment. Graphene quantum dots can be obtained in just one step, but their particle sizes are uneven.
Bottom up synthesis
The bottom-up approach refers to the preparation of graphene quantum dots using smaller structural units as precursors through a series of interaction forces, mainly through preparation pathways such as solution chemistry, ultrasound, and microwave methods.
The solution chemistry method is mainly used to prepare graphene quantum dots through the solution phase chemistry method of aryl oxidation condensation. The synthesis process involves the gradual condensation reaction of small molecule (3-iodo-4-bromoaniline or other benzene derivatives) polymers to obtain polystyrene dendritic precursors, by oxidation reaction to obtain graphene groups, and finally etching to obtain graphene quantum dots.
The microwave principle uses sugars (such as glucose, fructose, etc.) as carbon sources, because after dehydration, sugars can form C=C, which can form the basic skeleton unit of graphene quantum dots. The hydrogen and oxygen elements in hydroxyl and carboxyl groups will be dehydrated and removed in a hydrothermal environment, while the remaining functional groups will still bind to the surface of graphene quantum dots. They exist as passive layers, which can make graphene quantum dots have good water solubility and fluorescence properties.
The practical application of GQDs
Graphene quantum dots have important potential applications in fields such as biology, medicine, and new semiconductor devices. It can achieve single molecule sensors, and may also give rise to ultra small transistors or on-chip communication using semiconductor lasers for the production of chemical sensors, solar cells, medical imaging devices, or nanoscale circuits, among others.
 online service
online service 13929258449
13929258449 admin@satnano.com
admin@satnano.com + 8613929258449
+ 8613929258449