The sintering of materials involves at least two processes: densification of the body and growth of grains in the body.
The longevity of grains is usually achieved through the movement of grain boundaries. According to the classical theory of grain growth kinetics, the difference in free energy between the two sides of a curved grain boundary is the driving force that causes the interface to move towards the center of curvature. In the blank, most grain boundaries are curved. From the center of each grain, some grain boundaries are concave while others are convex. The interface energy of a convex surface is greater than that of a concave surface, so atoms or ions will transition from the convex surface to the concave surface, causing the grain boundary to move towards the center of curvature of the convex surface. The result is that some grains with concave grain boundaries grow, while others with convex grain boundaries shrink or disappear. The ultimate result is the growth of the average grain size. However, actual sintering is very complex. Taking grain growth as an example, there are other ways besides this classic method.
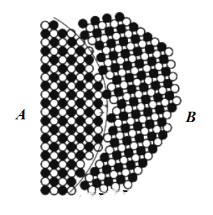
One method is to merge two adjacent grains into one large grain. In the formed blank, the orientation of each grain is random. In most cases, grain boundaries form at the neck due to the different orientations of adjacent grains. During the subsequent sintering process, grain boundaries migrate, with some grains growing and others becoming smaller or disappearing. But there are also some adjacent grains with consistent or almost consistent orientations. During sintering, the lattice of these grains will automatically match, the grain boundaries will disappear, and a continuous structure will be formed. Two small grains will grow into one large grain. Sometimes, two adjacent grains can also rotate to achieve matching.
The second is gas-phase transmission. The saturated vapor pressure on the surface of grains of different sizes is different. The finer the grains, the higher the saturated vapor pressure, and the easier it is for the material to vaporize. After gasification, the material is transported through the pores between grains and condensed on the surface of coarser grains, causing these grains to grow. An interesting phenomenon is that when grain growth mainly relies on gas-phase transport mechanisms, even if there is no densification of the billet during the process, the grains will still grow. For example, studies have found that sintering titanium oxide bodies in HCl steam results in an initial particle size of 0.2 microns and a density of only 45% after sintering, which is basically the same as the density of the green body. But at this point, the average size of titanium oxide grains has grown to 6 microns.
The third is liquid-phase transport. We know that a mass transfer process during liquid-phase sintering is dissolution precipitation. The dissolution precipitation mass transfer can be divided into two types. One is mass transfer on the same grain, which dissolves at the sharp points of the grain (or at the contact interface with other grains) and deposits on other flat surfaces of the grain through liquid phase transfer. The other is due to the uneven grain size inside the billet, which causes small grains to dissolve due to the difference in curvature between grains and deposit on larger grains through liquid phase transfer. The former mass transfer process only causes changes in grain shape, while the latter mass transfer process causes grain growth (accompanied by the disappearance of fine grains). At this point, the densification process of the billet is also a process of grain production.
However, the liquid-phase sintering process mentioned above has a prerequisite that the liquid phase can wet the solid phase. If wetting is not possible, although a liquid phase is formed during the sintering process, it can only be isolated between solid phases and cannot form a continuous phase to encapsulate the solid phase, making it difficult to form effective sintering. But under such conditions, the grains will still grow, relying not on dissolution precipitation mass transfer, but on the migration of solid solid grain boundaries.
SAT NANO is a best supplier of nanopowder and micron powder in China, we can supply product for sintering, we offer WC-Co powder, tungsten powder, tungsten carbide powder, MgO nanopowder, ZrO2 powder, if you have any enquiry, please feel free to contact us at admin@satnano.com
 online service
online service 13929258449
13929258449 admin@satnano.com
admin@satnano.com + 8613929258449
+ 8613929258449